Multidrug efflux mediated by ABC transporters
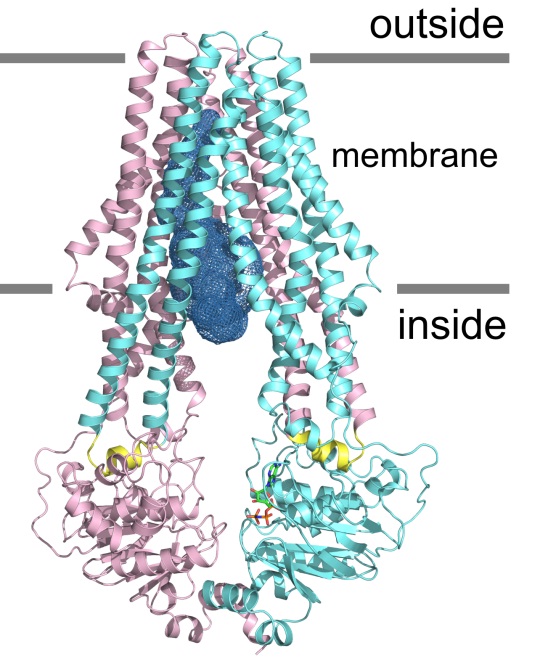
ABC transporters represent a large protein family found in every living cell ( Biochim. Biophys. Acta 2009, 1794:725-737, Chapter ABC Transporters - 40 Years on, pp 65-84). They are divided in ABC importers, which are exclusive to bacteria and ABC exporters present in all phyla of life. ABC exporters typically consist of two transmembrane domains (TMDs) responsible for substrate translocation, which are coupled to a pair of nucleotide binding domains (NBDs) that act as ATP-fuelled motor domains driving the transport cycle.
We shed light on the molecular organization of heterodimeric ABC exporters by solving the crystal structure of TM287/288, a multidrug transporter from the hyperthermophilic bacterium Thermotoga maritima. The first TM287/288 structure was obtained with a bound nucleotide (Nat. Struct. Mol. Biol. 2012, 19:396-402) and subsequently, we also published the structure of its apo state (PNAS 2014 Jul 29;111(30):11025-30.) and of its isolated motor domains (Biochemistry, Bukowska et al. 2015). TM287/288 represented the first structure of a heterodimeric ABC exporter with asymmetric ATP binding site and its conformational cycle was elucidated using electron paramagnetic resonance (EPR) techniques (eLife, Timachi et al 2017). Conformation-specific synthetic nanobodies (sybodies) inhibiting the ATPase activity of TM287/288 were generated using our recently developed sybody selection platform. One of these sybodies was used to solve the crystal structure of TM287/288 in its outward-facing state (Hutter et al., Nature Communications, 2019). A pair of nanobodies targetting the NBDs of TM287/288 were used as spin-labeled probes to investigate conformational cycling of TM287/288 in near-native membranes (Galazzo et. al, Proc Natl Acad Sci, 2020)
Recently, we identified a heterodimeric ABC exporter called EfrCD, which strongly contributes to drug efflux in the opportunistic pathogen Enterococcus faecalis [AAC, Hürlimann et al. 2016, FEBS J, Hürlimann et al. 2017]. We currently study EfrCD at the functional and structural level to identify molecular hallmarks of drug efflux pumps. To this end, we perform deep mutational scanning to obtain a global picture of residues playing a role in drug recognition and transport. Our long-term goal is to understand promiscuity of drug recognition and the mechanics of drug pumping at the molecular level.